Nedelec
Stem cells in neurodevelopment
We study human development and associated diseases with stem cell-based models of embryogenesis and organogenesis
NEWS
Our latest article “Self-organizing models of human trunk organogenesis recapitulate spinal cord and spine co-morphogenesis” is now out in Nature Biotechnology.
Research
Our lab is studying the molecular and cellular basis of human development in health and disease. To approach this question we use human pluripotent stem cell differentiation into organoids or specific cell types coupled to transcriptomic, optogenetic and live imaging approaches
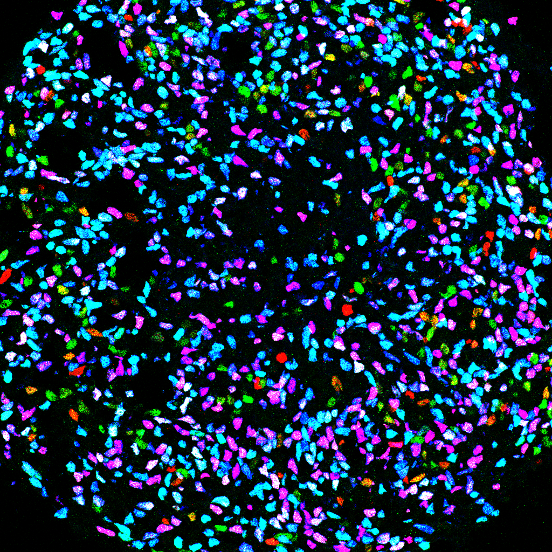
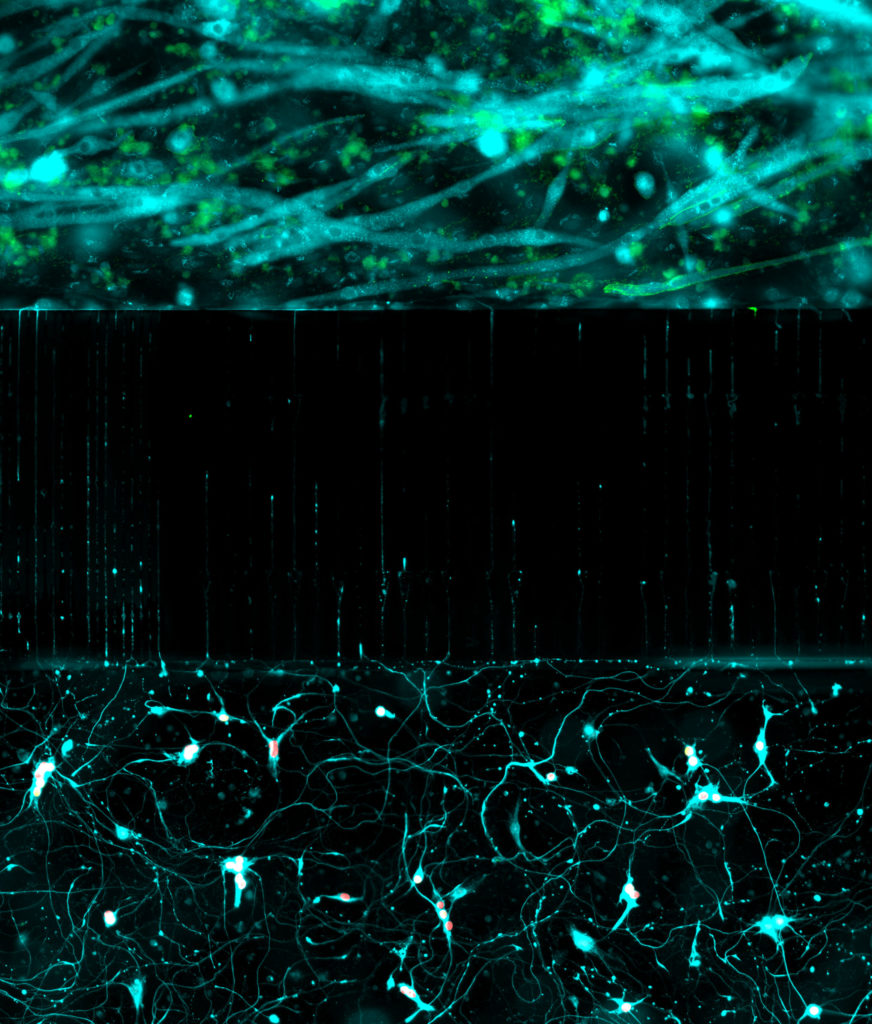
We seek to decode the principles controlling the specification and organization of neural diversity and how genetic mutations perturb these events to cause disorders. To approach these questions, we are creating in vitro models of human embryogenesis (embryoids, organoids and directed differentiation) based on the differentiation human induced pluripotent stem cells. These models are not only revolutionizing the way we address the mechanisms controlling the formation of organs and specific cell types, but also open avenues to produce defined cell populations or tissues (cell and tissue engineering) to study disease mechanims, for cell therapy strategies (transplantation) or drug screening.
Human development / cell and tissue engineering.
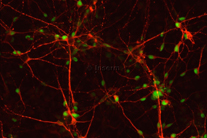
1) Using targeted differentiation of human pluripotent stem cells toward specific neuronal subtypes, we investigate the signal transduction pathways and genetic networks that ensure the formation of distinct locomotor neural circuits along the body axis. (Projects of Célia Vaslin and Rémi Robert, see also Nedelec, Martinez-Arias, Cur. Op. Neuro, 2021). Building on our previous work in which we invented a new way to investigate cell fate control using in vitro differentiation of human pluripotent stem cells (Maury et al. Nature Biotechnology, 2015), we have recently discovered mechanisms regulating HOX gene expression resulting in the first efficient methods to generate distinct motor neuron subtypes differentially impacted in motor neuron diseases (Mouilleau, Vaslin et al, Development, 2021). Rémi Robert, a graduate student in the group, continue to explore the links between extrinsic cues and the HOX timer using transcriptomic and live imaging of signaling reporters. Collaboration : Benoit Sorre (Institut Curie, Paris), Vanessa Ribes (IJM, Paris), Esteban Mazzoni (NYU, New York)
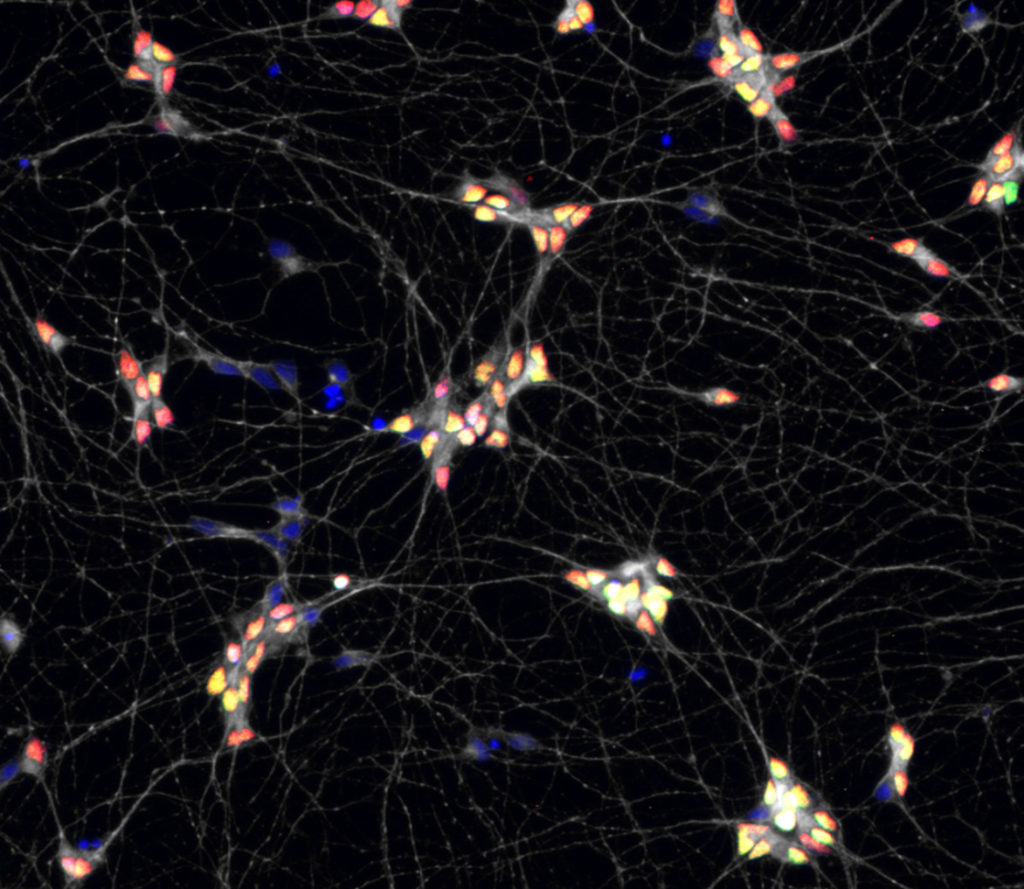
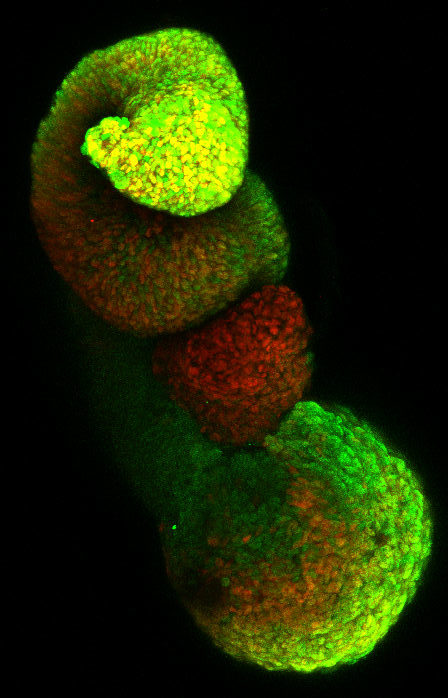
2) Using a new multi-lineage organoid model we have recently regenerated , we are studying the coupling between the morphogenesis of the neural tube and the spatial allocation of specific neuronal circuits. We combine live imaging, single cell and spatial transcriptomic to approach this question.
Collaboration Alexander van Oudenaarden lab (Hubrecht institute, Utrecht, NL) and Xavier Morin lab (ENS, Paris). FC3R and Ibio funded projects
Basis of neurodevelopmental and neurodegenrative disorders
These in vitro models provide access to human tissues or cell types affected in pathologies. We use patient-derived induced pluripotent stem cells to study the basis of motor neuron diseases, a heterogeneous group of incurable and often fatal diseases. In particular, we are studying infantile spinal muscular atrophies (SMAs) and juvenile ALS which, although caused by mutations in ubiquitously expressed genes, are characterized by defects in the formation or survival of particular populations of motor neurons while others are preserved. Deciphering the basis of the vulnerability or the resistance of these different types of motor neurons should open new therapeutic avenues
Projects :
1) Maeliss Calon, a graduate student cosupervised with Alexandre Baffet is investigating the deregulation of axonal transport and motor neuron subtype differential vulnerability in distal spinal muscular atrophies.
Collaboration with the Baffet lab (Curie), N. Bahi-Buisson (Imagine) and A. Rossor (UCL), C. Villard (IPGG, Paris). ANR and AFM funded program.
2) Marta Giannini, a post-doc, is studying the Molecular and cellular basis of a juvenile form of amyotrophic lateral sclerosis caused by Senataxin mutations.
Collaboration with Odil Porrua, IGGM, Montpellier.
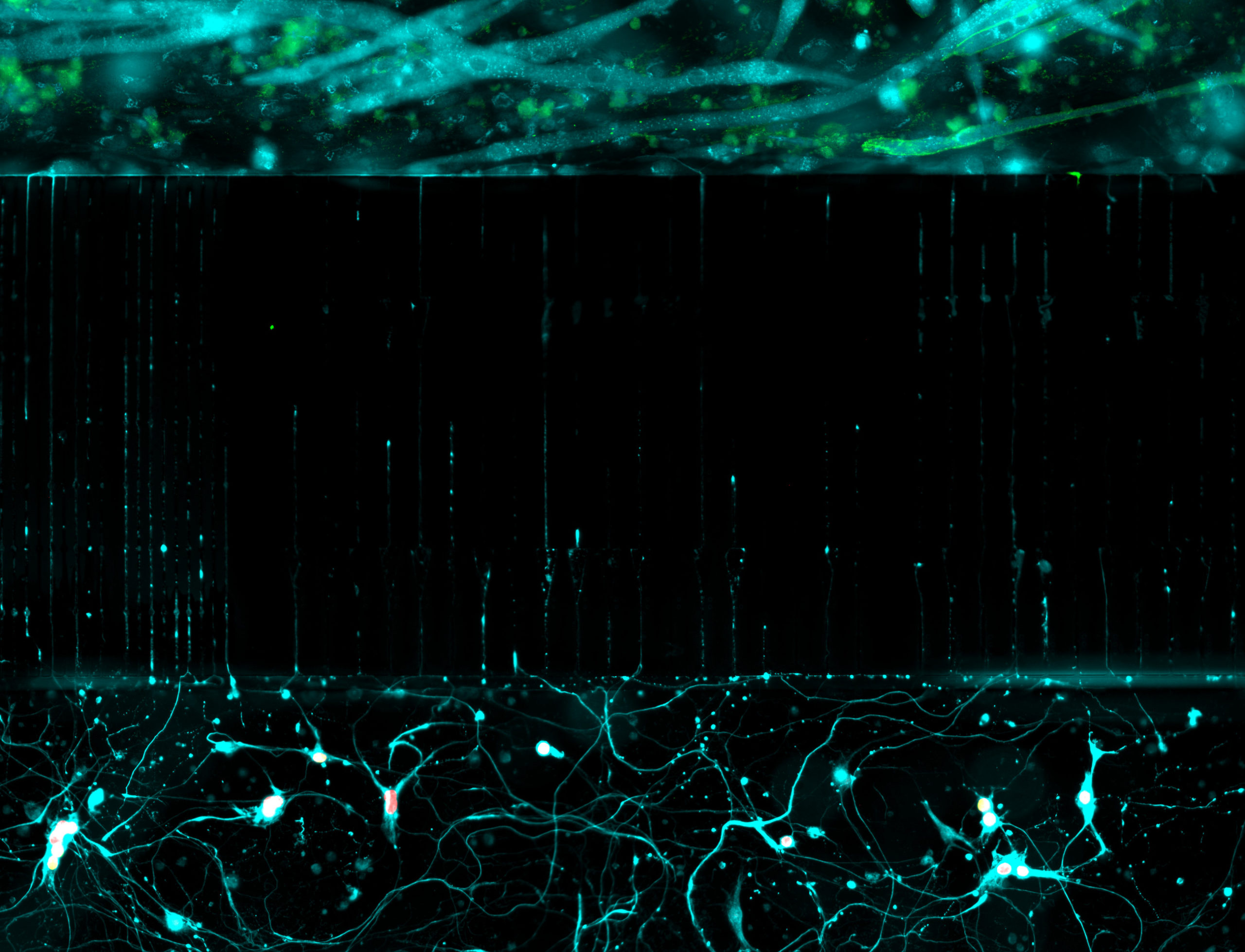
in a microfluidic chamber
Methods
- Human induced pluripotent stem cell (iPS)
- organoid
- Transcriptomics
- Microfluidic
- Histology
- Live imaging
People
Team leader : Stéphane NEDELEC, CRCN INSERM
- Simona Gribaudo, Research engineer, Sorbonne Université
- Rémi Robert, PhD student (Cosupervised with J. van Helden, ATGC, Marseille)
- Maeliss Calon, PhD student (Cosupervised with A. Baffet, Curie Institute)
- Marta Giannini, Post doctoral scientist (between O. Porrua and Nedelec labs)
Collaborators
- Ribes lab , IJM, Paris
- van Oudenaarden lab, Hubrecht, Utrecht
- Baffet lab, Curie Institute, Paris
- Nadia Bahi-Buisson, Imagine Institute, Paris
- Livet lab, institut de la vision, Paris
- Morin lab, ENS, Paris
- Alexander Rossor, UCL, London
- Mazzoni lab, NYU, New York,
- Wichterle lab, Columbia University, New York
- Sorre lab, Curie institute, Paris
- Villard lab, IPGG, Paris
- Porrua-Fuerte lab, IGMM, Montpellier
Funding
- ATIP/Avenir program – Inserm
- Association Francaise contre les Myopathies (AFM)
- Agence Nationale de la Recherche (ANR)
- Fondation pour la Recherche Médicale (FRM)
- ibio
- FC3R


